Publication – Automated generation and reactions of 3-hydroxymethylindoles in continuous-flow microreactors
Chem. Eur. J. 2010, 16, 6678 – 6686
- Dr. T. Tricotet, Prof. D. F. O’Shea
- School of Chemistry and Chemical Biology, University College Dublin, Dublin 4 (Ireland)
This synthesis was performed using the Syrris Africa system (replaced by Asia).
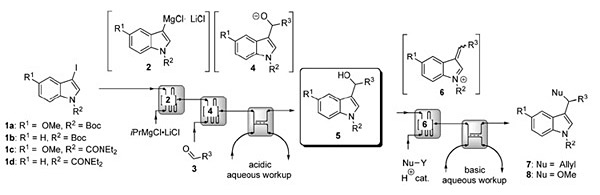
Abstract: An automated sequential approach for the generation and reactions of 3-hydroxymethylindoles in continuous-flow microreactors is described. Consecutive halogen–magnesium exchanges of four 3-iodoindoles followed by addition to three aldehydes provided twelve 3-hydroxymethylindoles in a multi-microreactor setup. The synthetic flow strategy could be coupled with an in line continuous liquid–liquid extraction workup protocol for each reaction. Further elaboration of each of these indoles within the fluidic setup was achieved by acid-catalyzed nucleophilic substitutions with allyltrimethylsilane and methanol used as nucleophiles.
Overall, a set of four 3-iodoindoles was converted into thirty-six indole derivatives by a range of transformations including iodo–magnesium exchange/electrophile trapping and acid-catalysed nucleophilic substitution in a fully automated sequential fashion.
This synthesis was performed using the Syrris Africa system (replaced by Asia).

