Publication – One-Step flow synthesis of substituted pyrrole-3-carboxylic acid derivatives via in situ hydrolysis of tert-butyl esters
Org. Lett., 2010, Vol. 12 (22)
- Ananda Herath and Nicholas D. P. Cosford*
- Sanford-Burnham Medical Research Institute, 10901 N. Torrey Pines Rd., La Jolla, CA 92037, USA
This paper describes the synthesis of pyrrole-3-carboxylic acids by a one-step continuous Hantzsch reaction where the hydrogen bromide by-product is used to hydrolyse the ester in-situ. The reaction has been performed in a Syrris glass microreactor on a Syrris Africa system (replaced by the Asia system in 2012) with a wide range of substrates in good yields and fast reaction times.
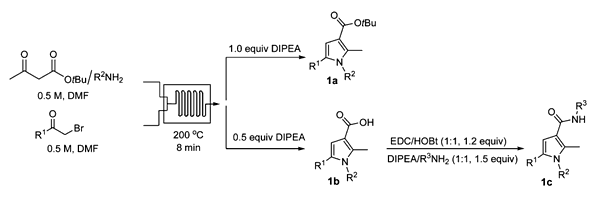
Abstract: The first one-step, continuous flow synthesis of pyrrole-3-carboxylic acids directly from tert-butyl acetoacetates, amines, and 2-bromoketones is reported. The HBr generated as a byproduct in the Hantzsch reaction was utilized in the flow method to hydrolyze the t-butyl esters in situ to provide the corresponding acids in a single microreactor. The protocol was used in the multistep synthesis of pyrrole-3-carboxamides, including two CB1 inverse agonists, directly from commercially available starting materials in a single continuous process.
This paper was performed on the Africa system (replaced by Asia in 2012).

