Benefits of flow photochemistry
The benefits of flow chemistry over traditional batch techniques are widely known; all of these translate equally to flow photochemistry. Most of these benefits come from the precise control of reaction parameters that flow chemistry offers. By controlling factors such as temperature, mixing, stoichiometry, and reaction times, we can enhance the control of our chemical reactions.
In a flow photochemistry reactor, we can increase this control further over traditional batch photochemical techniques:
The benefits of using flow photochemistry techniques vs. batch photochemical techniques are explained in detail below.
-
Improved reaction control
The benefits of flow chemistry over traditional batch techniques are widely known and translate equally to flow photochemistry. Most of these benefits come from the precise control of reaction parameters that flow chemistry offers. By controlling factors such as temperature, mixing, stoichiometry, and reaction times, we can enhance the control of our chemical reactions. In a flow photochemistry reactor, we can increase this control further over traditional batch photochemical techniques.
Flow photochemistry reactors offer:
Consistent light penetration: this homogeneity allows reproducible reactions
Controlled reaction exposure times: reducing degradation of products and unwanted side reactions
The ability to increase photon flux into the reactor: potentially increasing reaction rates
A simpler route to scale-up: increasing reaction rates can help increase reaction throughput
If we consider the use of monochromatic LED light sources, we can add better reaction selectivity through the control of the wavelength used, enabling higher conversions and yields, and better heat exchange via isolation of the light source temperature and reactor.Access to Multiphase Chemistry
The design of flow chemistry reactor systems allows chemists to perform reactions in numerous ways. The most common is homogeneous chemistry, where all the reactants and products are in solution. Reactions can also be performed in multiphase environments. We can carry out reactions with liquid-liquid partitions, where the two liquids are immiscible and also in liquid-gas partitions, where one of the components is a gas.The size of flow reactors is typically small, which enables the ability to easily pressurize the reactor and flow system. This has a multitude of benefits:
we can increase the temperature of a reaction above its reflux temperature
we can avoid cavitation of low boiling point and low vapour pressure reactants
we can introduce gases into the flow reaction
It is the final point that is the most relevant to photochemical applications. Gas insertion type reactions such as continuous oxidations, hydrogenation, halogenations and carbonylations are well documented.1The use of oxygen in photochemistry for photooxygenation chemistries is an important application. This is highlighted by the synthesis of the anti-malaria drug Artemisinin demonstrated by the Seeberger group.3
Molecular oxygen is an attractive oxidant due to its atom efficiency, reduced cost, and sustainable nature. However, these reactions are prone to over oxidations, which leads to to unwanted by-products.
Flow photochemistry with its ability to control the reaction exposure time can offer an advantage over traditional batch photochemistry techniques.
In these applications, singlet oxygen (1O2) can be produced starting from triplet oxygen (3O2), by light irradiation in the presence of a suitable photosensitizer. Working with large amounts of oxygen gas in batch conditions is extremely hazardous due to its flammable nature. Flow photochemistry can provide easy access to 1O2 in a much safer process, compared with batch procedures. 1O2 exhibits higher electrophilicity compared with 3O2, which allows substrates to be oxidized that are otherwise unreactive to oxygen.3
For more information about flow photochemistry or how you can achieve better results using Syrris products, please contact us.
Contact us to discuss your lab’s flow photochemistry needs
1. Cambié, D.; Bottecchia, C.; Straathof, N. J. W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev., 2016, 116 (17), 10276–10341. https://doi.org/10.1021/acs.chemrev.5b00707
3. Lévesque, F.; Seeberger, P. H. Continuous-Flow Synthesis of the Anti-Malaria Drug Artemisinin. Angew. Chemie Int. Ed., 2012, 51 (7), 1706–1709. https://doi.org/10.1002/anie.201107446.
-
Access to multiphase chemistry
The design of flow chemistry reactor systems allows chemists to perform reactions in numerous ways. The most common is homogeneous chemistry, where all the reactants and products are in solution. Reactions can also be performed in multiphase environments. We can carry out reactions with liquid-liquid partitions, where the two liquids are immiscible and also in liquid-gas partitions, where one of the components is a gas.
The size of flow reactors is typically small, which enables the ability to easily pressurize the reactor and flow system. This has a multitude of benefits:
- we can increase the temperature of a reaction above its reflux temperature
- we can avoid cavitation of low boiling point and low vapour pressure reactants
- we can introduce gases into the flow reaction
It is the final point that is the most relevant to photochemical applications. Gas insertion type reactions such as continuous oxidations, hydrogenation, halogenations and carbonylations are well documented.1
The use of oxygen in photochemistry for photooxygenation chemistries is an important application. This is highlighted by the synthesis of the anti-malaria drug Artemisinin demonstrated by the Seeberger group.3
Molecular oxygen is an attractive oxidant due to its atom efficiency, reduced cost, and sustainable nature. However, these reactions are prone to over oxidations, which leads to to unwanted by-products.
Flow photochemistry with its ability to control the reaction exposure time can offer an advantage over traditional batch photochemistry techniques.
In these applications, singlet oxygen (1O2) can be produced starting from triplet oxygen (3O2), by light irradiation in the presence of a suitable photosensitizer. Working with large amounts of oxygen gas in batch conditions is extremely hazardous due to its flammable nature. Flow photochemistry can provide easy access to 1O2 in a much safer process, compared with batch procedures. 1O2 exhibits higher electrophilicity compared with 3O2, which allows substrates to be oxidized that are otherwise unreactive to oxygen.3
-
Improved irradiation of the reaction
Historically, photochemistry uses high intensity mercury lamps to facilitate photocatalyzed reactions. These light sources come with significant difficulties in their operation. Mercury lamps produce a broad spectrum of wavelength with strong emissions in the UV spectrum. In medium pressure mercury-vapor lamps, the wavelength range emitted is from 200–600 nm. Therefore, to offer specific and selective photocatalyzed reactions, a suitable filter is require to block unwanted wavelengths. These filters will typically block either shorter wavelengths (long-pass filters) or allow specific wavelengths to pass (band-pass filters). Undesired wavelengths can cause unwanted side-reactions and the potential decomposition of products.
Low and medium pressure mercury lamps produce a lot of heat. This requires external cooling to be implemented and to ensure that the heat the lamps produce is isolated from the photochemistry reactor to prevent this interfering with the desired reaction.
One of the most serious implications of using mercury lamps is the high level of ionising radiation they emit. Specific features need to be implemented to prevent the unwanted exposure of users to UV light which can be extremely harmful resulting in sun burn and extreme damage to the eyes.
The use of compact fluorescent light bulbs (CFL) has gained some use for visible light-induced reactions which can emit a broad range of wavelengths, but they have limitations when applied to flow photochemistry reactors due to the broad area of irradiation resulting in loss of light directed towards the reaction.
The continued development of light emitting diode (LED) technology is driving their use in flow photochemistry reactors. LED light sources typically have a narrow emission band (normally in 20nm ranges) so can be selected to match the specific photochemical application. They also have a small irradiation window allowing them to be directed towards the channels in flow reactors. LED light sources also have a low heat load enabling them to be used at high intensities with relatively low cooling required and are energy efficient. All of these benefits have seen the rise in popularity of their use in photochemistry reactors.
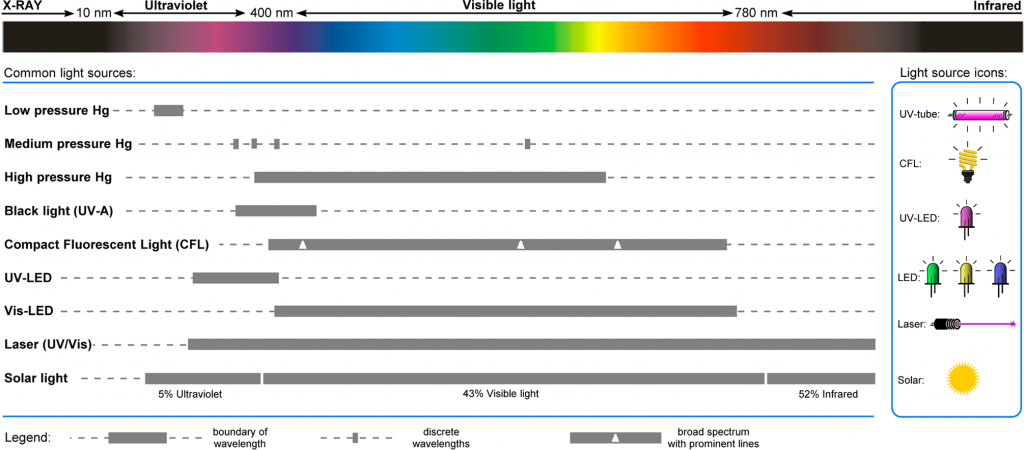 Figure 1 – Emission Spectra of Common light sources used for Photochemical Applications1
Figure 1 – Emission Spectra of Common light sources used for Photochemical Applications1For more information about flow photochemistry or how you can achieve better results using Syrris products, please contact us.
Contact us to discuss your lab’s flow photochemistry needs
1 Cambié, D.; Bottecchia, C.; Straathof, N. J. W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev., 2016, 116 (17), 10276–10341. https://doi.org/10.1021/acs.chemrev.5b00707
Related products
-
Improved irradiation to the reactor
Photochemistry reactions are activated by the adsorption of photons (discrete “packets” of energy) by a reagent in a reaction. To put it more simply, photochemical reactions occur when light provides energy to trigger a reaction. The problem with photons is the attenuation effect of photon transport, that is they lose their energy very quickly. This is described by the well-known Bouguer-Lambert-Beer law which explains that due to absorption effects the radiation distribution is not uniform. For a photocatalyzed reaction, a homogeneous condition is ideal for better yields and selectivity.
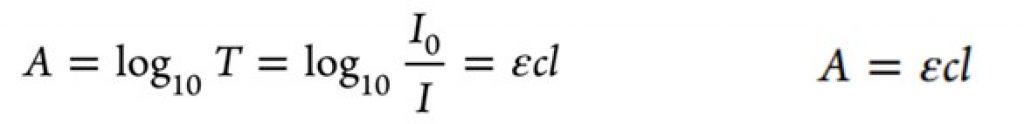 Equation 1 – Bouguer−Lambert−Beer law equation1Bouguer−Lambert−Beer law equation relating the molar extinction coefficient (ε) of the light absorbing molecules, their concentration (c), and the path length of light propagation1
Equation 1 – Bouguer−Lambert−Beer law equation1Bouguer−Lambert−Beer law equation relating the molar extinction coefficient (ε) of the light absorbing molecules, their concentration (c), and the path length of light propagation1There are several factors illustrated in the equation. The concentration of the reaction (c) plays a role in the light absorption with the pathlength of the light propagation (l), or the distance of the light source from the reaction mixture also an important factor.
Even the reaction mixture can effect attenuation. Starting materials, products, photosensitizers, and photocatalysts can all act as filters reducing the light intensity.
An example of how the attenuation of light affects a reaction is shown below. Here the % transmittance of a common photocatalyst, tris(bipyridine)- ruthenium(II) chloride [Ru(bpy)3]2+, is plotted against the path length for different concentrations (Figure 2). For a typical catalyst concentration (e.g. 2.5 mM), Figure 2 shows that less than 0.1% of light is transmitted past a length of 0.1 cm from the point of incident light and that even in a 1 cm vial, the majority of the reaction mixture is not being irradiated. Even after reducing the catalyst concentration 10-fold (0.25 mM), there would be ∼1% of the incident light transmitted to the centre of the reaction vessel. Unfortunately, by lowering the concentration of the catalyst, we also lower the rate of reaction.
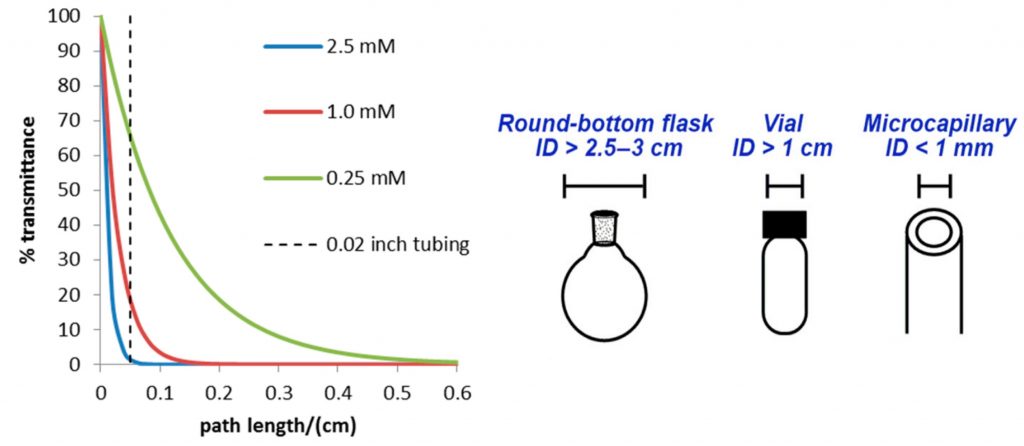 Figure 2 – Attenuation of light with the distance of irradiation4, 5
Figure 2 – Attenuation of light with the distance of irradiation4, 5When we look at performing photochemical reactions in microchannels (with an internal diameter of < 1 mm), we can see that full transmission of light is allowed through to the reaction solution. This results in a higher and more homogeneous irradiation of the reaction giving shorter reaction times and consequently less side-products caused by over-irradiation, often seen in equivalent batch conditions. This has been the major driving force behind the development of flow photochemistry reactors. By using well established flow chemistry reactors, the transmission of light is greatly increased. There are some important factors to consider when selecting the material of the flow photochemistry reactor. It is well known that certain materials can act as a wavelength filter, much like the reactants. Therefore, it is essential that the walls of the reactor are transparent to the frequency of light that is being used to irradiate the process.
Glass is an excellent example of a light transparent material. However, some types of glass can be incompatible with strong acid conditions. Photochemistry reactors have been increasingly made from polymer materials such as perfluoroalkoxyalkane (PFA) or polytetrafluoroethylene (PTFE). These materials have excellent light transmission, are highly chemically inert and have good thermal properties. Both PFA and FEP tubing have very good light transmission in the ultraviolet and visible light regions and allow a flexible configuration to flow photochemistry reactors. Figure 3 shows the cut-off wavelengths for a number of different materials that can be used within photochemical reactors.
Reactor material Wavelength cut-off Quartz glass 170 nm Vycor glass 220 nm Corex glass 260 nm Pyrex glass 275 nm Polymethylmethacryate (PMMA) 248 nm Polydimethylsiloxane (PDMS) ±255 nm Polytetrafluoroethylene (PTFE) ±200 nm Perfluoroalkoxyalkane (PFA) ±180 nm Perfluoroethylenepropylene (FEP) ±180 nm Figure 3 – Wavelength cut-off of materials used in flow photochemistry reactors1
For more information about flow photochemistry or how you can achieve better results using Syrris products, please contact us.
Contact us to discuss your lab’s flow photochemistry needs
1 Cambié, D.; Bottecchia, C.; Straathof, N. J. W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev., 2016, 116 (17), 10276–10341. https://doi.org/10.1021/acs.chemrev.5b00707
2 Knowles, J. P.; Elliott, L. D.; Booker-Milburn, K. I. Flow Photochemistry: Old Light through New Windows. Beilstein J. Org. Chem., 2012, 8, 2025–2052. https://doi.org/10.3762/bjoc.8.229
4 Sambiagio, C.; Noël, T. Flow Photochemistry: Shine Some Light on Those Tubes! Trends Chem., 2020, 2 (2), 92–106. https://doi.org/10.1016/j.trechm.2019.09.003
5 Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev., 2017, 117 (18), 11796–11893. https://doi.org/10.1021/acs.chemrev.7b00183
-
Photochemistry reaction scalability
It could be argued that the popularity of photochemistry amongst synthetic organic chemists in industry has suffered from its limited scale-up potential. This can be attributed to the attenuation effect of photon transport. The intensity of the energy rapidly decreases the further it is away from the reaction, as described by the Bouguer-Lambert-Beer law. With the increase in reactor volume, this effect is enhanced leading to poor light transmission to the reaction mixture. This limits the scalability of photochemistry even on a laboratory scale under batch conditions. This effect also limits the classical way in which flow chemistry is often scaled, by increasing reactor volumes and flow rates. Reactor volumes are often increase in a dimension-enlarging fashion, where the attenuation of light also restricts this scale-up method. Flow photochemistry has a couple of strategies that can help in the scale up of photocatalytic reactions.
We can increase the throughput by running the reactions for longer:
- We can increase the number of reactors
- We can increase the photon flux into the reaction
The first method is well known as a benefit of traditional flow chemistry. We can use the same reactor to produce more material by increasing the reaction time. The second point is to number-up reactors and can be achieved in several ways. Linear numbering-up is where several reactors are placed along the fluidic pathway to increase the volume, and subsequent flow rate and through put. External numbering-up is where the entire flow system is duplicated and internal numbering-up is where multiple reactors are fed from the same pump source with the fluid channels split into multiple streams. External numbering-up is clearly a more expensive option. Internal numbering-up can lead to irregular residence times across the reactors. Internal numbering up is the better option as we can maintain the same light conditions within each reactor, but this is also a costly approach.
It is useful to consider here a light source as emitting a flux of photons. An equation to calculate the number of moles of photons (einsteins) per hour at a given wavelength (λ) (if the total power of emissions at that wavelength is known), is shown in Equation 2.
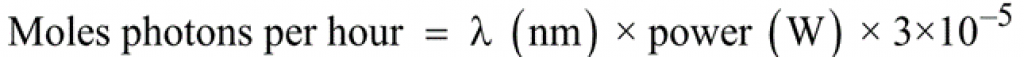 Equation 2 – Number of moles of photons (einsteins) per hour at a given wavelength (λ)2
Equation 2 – Number of moles of photons (einsteins) per hour at a given wavelength (λ)2If we compare batch and flow photochemistry techniques, flow photochemistry reactors have been shown to deliver around a 150-fold higher absorbed photon flux density compared to their batch equivalent. Photon flux is calculated by dividing the photon flux (einsteins/s) by the reactor volume. The photon flux strongly affects the reaction rate of photochemical processes; the higher the photon flux, the faster the reaction will be completed.1 This explains clearly why photochemical reactions can be substantially accelerated in microreactors.
As we consider the photon as a reagent in photochemistry it makes sense that by increasing the number of photons into a photo mediated reaction could increase the reaction rate. If we can increase the light intensity into the same photochemistry reactor then we can use the same platform to scale-up our reactions.
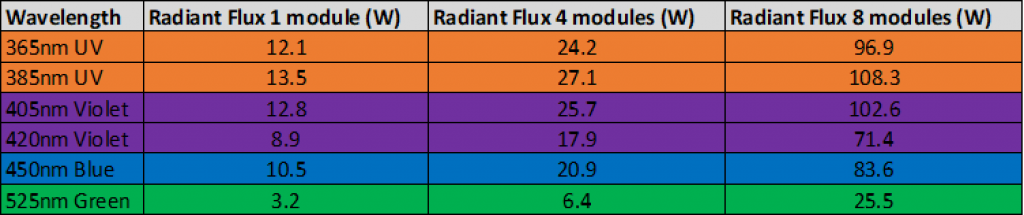 Figure 4Table showing the increased radiant flux delivered by the Asia Photochemistry Reactor by increasing LED modules for scaling photochemistry reaction.
Figure 4Table showing the increased radiant flux delivered by the Asia Photochemistry Reactor by increasing LED modules for scaling photochemistry reaction.For more information about flow photochemistry or how you can achieve better results using Syrris products, please contact us.
Contact us to discuss your lab’s flow photochemistry needs
1 Cambié, D.; Bottecchia, C.; Straathof, N. J. W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev., 2016, 116 (17), 10276–10341. https://doi.org/10.1021/acs.chemrev.5b00707
2 Knowles, J. P.; Elliott, L. D.; Booker-Milburn, K. I. Flow Photochemistry: Old Light through New Windows. Beilstein J. Org. Chem., 2012, 8, 2025–2052. https://doi.org/10.3762/bjoc.8.229

